Baltic amber is fascinating biogenic gemstone
Full of secrets and mystery. It has been the subject of intensive research in a wide variety of scientific disciplines for more than 500 years!
There is no complete information about the structure, composition and structure of Baltic amber.
What we know?
The first described important chemical research on amber was conducted by Georgius Agricola in the 16th century. He carried out a dry distillation of amber. He obtained a very important product of this process, later recognized as succinic acid.
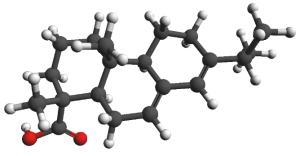
The main chemical elements of succinite are carbon (C), oxygen (O), hydrogen (H)
Research has also shown the presence of chemical elements: sulfur (S), nitrogen (N), iron (Fe), copper (Cu), sodium (Na), calcium (Ca), magnesium (Mg), manganese (Mn), aluminium (Al), nickel (Ni), silicon (Si).
The content of chemical elements is variable.
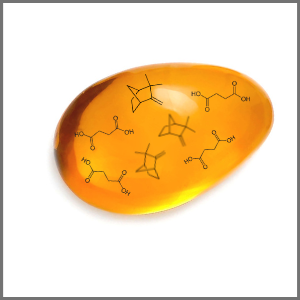
With Gas Chromatography (GC) and Mass Spectrometry (MS) ~50 chemical compounds can be separated from Baltic amber.
The chemical structure of amber is not homogenous.
The structure is based on two phases:
- micromolecular (soluble)
- macromolecular (insoluble)
Macromolecular skeleton is composed of polymerized and partially condensed combinations of compounds of the primary nature of diterpenoids.
In the free spaces of the structure there are monoterpenoids, sesquiterpenoids, free succinic acid and its esters, diterpenoids and products of diagenesis. These can be obtained for research by solvent extraction.
Baltic amber contains 3-8% of succinic acid
It occurs in free form and is bound by chemical and possibly physical bonds in the structure of succinite.